A water sample tested positive for lead with a concentration of 35 ppm.The density of the solution is 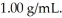 Which of the following statements is correct?
Which of the following statements is correct?
A) 100 g of the solution contains 35 g of lead
B) there are 35 mg of lead in 1.0 L of this solution
C) 100 g of the solution contains 35 mg of lead
D) the solution is 35% by mass of lead
E) the molarity of the solution is 35 M
Correct Answer:
Verified
Q130: The ideal value of i (van't Hoff
Q131: What is the molality of a 36.1%
Q132: What is the mole fraction of phosphoric
Q133: What is the molality (m)of a solution
Q134: Which produces the greatest number of ions
Q136: How many grams of solution are present
Q137: Which of the following will have an
Q138: A solution is prepared by dissolving 2.00
Q139: What is the mole fraction of nitric
Q140: How much sodium nitrate (g)is in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents