What is the vapor pressure (mm Hg) of water at 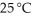 above a solution prepared by dissolving
above a solution prepared by dissolving 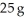 of urea,CO(NH2) 2,in
of urea,CO(NH2) 2,in 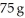 of water? The vapor pressure of pure water at 25 °C is 23.8 mm Hg.
of water? The vapor pressure of pure water at 25 °C is 23.8 mm Hg.
A) 22
B) 2.9
C) 0.42
D) 27
E) 0.91
Correct Answer:
Verified
Q123: Of the following,a 0.2 M aqueous solution
Q124: What is the molarity of phosphoric acid
Q125: An acetic acid aqueous solution contains 22%
Q126: What is the mole fraction of hydrochloric
Q127: Which produces the greatest number of ions
Q129: How many grams of solution are present
Q130: The ideal value of i (van't Hoff
Q131: What is the molality of a 36.1%
Q132: What is the mole fraction of phosphoric
Q133: What is the molality (m)of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents