The reaction CH3-N≡C → CH3-C≡N
Is a first-order reaction.At 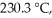
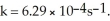 If
If 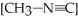 is 1.00 × 10-3 initially,
is 1.00 × 10-3 initially, 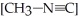 is ________ M after
is ________ M after 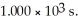
A) 5.33 × 10-4
B) 2.34 × 10-4
C) 1.88 × 10-3
D) 4.27 × 10-3
E) 1.00 × 10-6
Correct Answer:
Verified
Q6: The rate constant of a first-order process
Q7: The following reaction is second order in
Q8: One difference between first- and second-order reactions
Q9: Which one of the following is not
Q10: The magnitude of the rate constant is
Q12: Under constant conditions,the half-life of a first-order
Q13: Which of the following is correct regarding
Q14: What is the order of the reaction
Q15: The rate law of a reaction is
Q16: What is the magnitude of the rate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents