At Elevated Temperatures,methylisonitrile (CH3NC)isomerizes to Acetonitrile (CH3CN): CH3NC (G)→ CH3CN
At elevated temperatures,methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g)
The reaction is first order in methylisonitrile.The attached graph shows data for the reaction obtained at 198.9 °C. 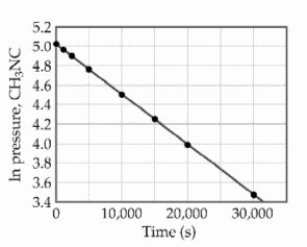 What is the rate constant (s-1) for the reaction?
What is the rate constant (s-1) for the reaction?
A) -1.9 × 104
B) +5.2 × 10-5
C) +1.9 × 104
D) -5.2 × 10-5
E) +6.2
Correct Answer:
Verified
Q23: The decomposition of [A] in solution at
Q24: Of the following,_ will lower the activation
Q25: The enzyme _ converts nitrogen into ammonia.
A)oxygenase
B)hydrogenase
C)oxidase
D)nitroglycerine
E)nitrogenase
Q26: The rate of a reaction depends on
Q27: As the temperature of a reaction is
Q29: Which of the following is true?
A)If we
Q30: A possible mechanism for the overall reaction
Q31: The primary source of the specificity of
Q32: In the energy profile of a reaction,the
Q33: In the Arrhenius equation, k = Ae-Ea/RT
_
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents