The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 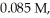 it takes
it takes  for it to decrease to 0.055 M.
for it to decrease to 0.055 M.
A) 8.2
B) 11
C) 3.6
D) 0.048
E) 8.4
Correct Answer:
Verified
Q57: What is the concentration (M)of S2O82- remaining
Q58: How many moles of B are present
Q59: The average rate of disappearance of I-
Q60: The average rate disappearance of A between
Q61: It was determined experimentally that the reaction
Q63: If the rate law for the reaction
Q64: The graph shown below depicts the relationship
Q65: The following reaction occurs in aqueous solution:
Q66: It was determined experimentally that the reaction
Q67: What is the order of the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents