Consider the following reaction at equilibrium: 2NH3 (g)  N2 (g) + 3H2 (g)
N2 (g) + 3H2 (g)
Le Châtelier's principle predicts that the moles of 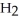 in the reaction container will increase with ________.
in the reaction container will increase with ________.
A) some removal of 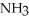 from the reaction vessel (V and T constant)
from the reaction vessel (V and T constant)
B) a decrease in the total pressure (T constant)
C) addition of some 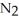 to the reaction vessel (V and T constant)
to the reaction vessel (V and T constant)
D) a decrease in the total volume of the reaction vessel (T constant)
E) an increase in total pressure by the addition of helium gas (V and T constant)
Correct Answer:
Verified
Q21: Based on Le Châtelier's principle,increasing pressure at
Q22: Consider the following reaction at equilibrium. 2CO2
Q23: The effect of a catalyst on an
Q24: The equilibrium constant for the gas phase
Q25: In which of the following reactions would
Q27: Which reaction will shift to the left
Q28: The equilibrium constant for the gas phase
Q29: How is the reaction quotient used to
Q30: The equilibrium constant for the gas phase
Q31: The equilibrium constant for the gas phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents