Multiple Choice
Dinitrogentetraoxide partially decomposes into nitrogen dioxide.A 1.00-L flask is charged with 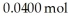 of N2O4.At equilibrium at 373 K,0.0055 mol of N2O4 remains.Keq for this reaction is ________.
of N2O4.At equilibrium at 373 K,0.0055 mol of N2O4 remains.Keq for this reaction is ________.
A) 2.2 × 10-4
B) 13
C) 0.22
D) 0.87
E) 0.022
Correct Answer:
Verified
Related Questions
Q37: The expression for Q38: Which of the following statements is true? Q39: The equilibrium constant for the gas phase Q40: The value of Keq for the equilibrium Q41: At 200 °C,the equilibrium constant (Kp)for the![]()
A)Q
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents