In the gas phase reaction below,NH3 is acting as a(n) ________. 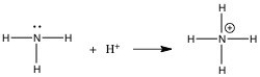
A) Br∅nsted-Lowry acid
B) Br∅nsted-Lowry base
C) Lewis base
D) Lewis acid
E) Arrhenius acid
Correct Answer:
Verified
Q42: The conjugate base of CH3NH3+ is _.
A)CH3NH2+
B)CH3NH2-
C)CH3NH+
D)CH3NH2
E)none
Q43: What is the conjugate acid of HCO3-?
A)CO22-
B)H2CO3
C)HCO22-
D)CO32-
E)none
Q44: Calculate the pH of a solution at
Q45: Of the following substances,an aqueous solution of
Q46: The conjugate base of HSO4- is _.
A)H2SO4
B)HSO4+
C)H+
D)SO42-
E)HSO3+
Q48: A 0.5 M solution of _ has
Q49: The conjugate acid of SO42- is _.
A)OH-
B)H2SO4
C)HSO4-
D)HSO42-
E)H3SO4+
Q50: What is the conjugate acid of OH-?
A)O2
B)H2O
C)O-
D)O2-
E)H3O+
Q51: What is the pH of an aqueous
Q52: Calculate the concentration (in M)of hydronium ions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents