The acid-dissociation constants of phosphoric acid (H3PO4) are 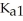 = 7.5 × 10-3,
= 7.5 × 10-3, 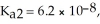 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
A) 1.82
B) 0.40
C) 2.51
D) 0.86
E) 0.13
Correct Answer:
Verified
Q74: The acid-dissociation constants of sulfurous acid (H2SO3)are
Q75: Determine the pH of a 0.35 M
Q76: A 0.14 M aqueous solution of the
Q77: An aqueous solution contains 0.390 M HCl
Q78: An aqueous basic solution has a concentration
Q80: A 0.22 M aqueous solution of the
Q81: Ka for arsenic acic,HAsO42-,is 7.5 × 10-12.What
Q82: Calculate the molarity of hydroxide ion in
Q83: What is the pH of an aqueous
Q84: In acidic solution,_.
A)[H3O+] > [OH-]
B)[H3O+] = [OH-]
C)[H3O+]
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents