The base-dissociation constant,Kb,for an unknown base is 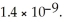 The acid-dissociation constant,Ka,for the conjugate ion is ________.
The acid-dissociation constant,Ka,for the conjugate ion is ________.
A) 1.0 × 10-7
B) 7.1 × 10-6
C) 1.4 × 10-23
D) 1.4 × 10-5
E) 7.1 × 10-4
Correct Answer:
Verified
Q82: Calculate the molarity of hydroxide ion in
Q83: What is the pH of an aqueous
Q84: In acidic solution,_.
A)[H3O+] > [OH-]
B)[H3O+] = [OH-]
C)[H3O+]
Q85: Ka for HA is 4.9 × 10-10.What
Q86: Which solution below has the highest concentration
Q88: What is the pH of an aqueous
Q89: The conjugate base of H2PO4- is _.
A)H3PO4
B)HPO42-
C)PO43-
D)H3O+
E)OH-
Q90: The Ka for HCN is 4.9 ×
Q91: The conjugate base of NH3 is _.
A)NH2-
B)NH4+
C)NH2OH
D)H3O+
E)OH-
Q92: An aqueous solution at 25.0°C contains [
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents