The acid-dissociation constant at 25.0 °C for hypochlorous acid (HClO) is 3.0 × 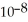 .At equilibrium,the molarity of
.At equilibrium,the molarity of 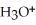 in a 0.066 M solution of HClO is ________.
in a 0.066 M solution of HClO is ________.
A) 4.4 × 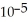
B) 0.066
C) 2.2 × 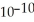
D) 4.35
E) 1.18
Correct Answer:
Verified
Q98: The pH of a 0.15 M aqueous
Q99: An aqueous solution of ammonia at 25.0
Q100: What is the pOH of an aqueous
Q101: In which of the following aqueous solutions
Q102: Which of the following salts will produce
Q104: Which of the following salts will produce
Q105: Hydrochloric acid is a strong acid.This means
Q106: The Ka of some acid,HA,at 25.0 °C
Q107: Which of the following 0.5 M aqueous
Q108: The ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents