Multiple Choice
Which of the following 0.5 M aqueous salt solutions will have a pH of 7.0 at 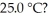 LiF RbBr NaClO4 NH4Cl
LiF RbBr NaClO4 NH4Cl
A) LiF only
B) NaClO4 only
C) LiF and RbBr
D) RbBr and NaClO4
E) NH4Cl only
Correct Answer:
Verified
Related Questions
Q102: Which of the following salts will produce
Q103: The acid-dissociation constant at 25.0 °C for
Q104: Which of the following salts will produce
Q105: Hydrochloric acid is a strong acid.This means
Q106: The Ka of some acid,HA,at 25.0 °C

