The 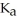 for acid HA is
for acid HA is 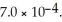 What is the pH of a 0.15 M aqueous solution of KA?
What is the pH of a 0.15 M aqueous solution of KA?
A) 0.82
B) 8.17
C) 5.83
D) 1.17
E) 5.01
Correct Answer:
Verified
Q127: What is the pOH of 0.606 M
Q128: What is the pH of a sodium
Q129: The pH of a 0.25 M aqueous
Q130: Of the following,which is the strongest acid?
A)HIO4
B)HIO3
C)HIO2
D)HIO
E)The
Q131: A Lewis acid is an electron-pair acceptor,and
Q133: The pOH of a 0.25 M aqueous
Q134: What is the pH of a 0.40
Q135: What is the pH of 0.626 M
Q136: Of the following,which is the weakest acid?
A)HPO3-
B)H3PO4
C)H2PO4-
D)HPO4-
E)The
Q137: The ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents