Multiple Choice
The 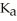 for formic acid (HCO2H) is 1.8 ×
for formic acid (HCO2H) is 1.8 × 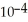 .What is the pH of a 0.20 M aqueous solution of sodium formate (NaHC
.What is the pH of a 0.20 M aqueous solution of sodium formate (NaHC 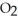 ) ?
) ?
A) 11.64
B) 5.48
C) 3.39
D) 8.52
E) 4.26
Correct Answer:
Verified
Related Questions
Q118: An aqueous solution contains 0.10 M HNO3.
Q119: In which of the following aqueous solutions
Q120: A 1.0 × 10-2 M aqueous solution
Q121: An acid containing the COOH group is
Q122: What is the pOH of a sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents