A 25.0 mL sample of 0.723 M HClO4 is titrated with a 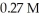 KOH solution.The H3O+ concentration after the addition of
KOH solution.The H3O+ concentration after the addition of 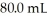 of KOH is ________ M.
of KOH is ________ M.
A) 0.4
B) 1 × 10-7
C) 0.7
D) 3 × 10-13
E) 4 × 10-2
Correct Answer:
Verified
Q17: The _ and _ are the principal
Q18: Which one of the following pairs cannot
Q19: The addition of KOH and _ to
Q20: A solution containing which one of the
Q21: The pH of a solution prepared by
Q23: A 25.0 mL sample of a solution
Q24: Which compound listed below has the greatest
Q25: Which one of the following is not
Q26: Human blood is considered to be _.
A)neutral
B)very
Q27: The Ka of benzoic acid is 6.30
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents