Multiple Choice
Calculate the pH of a solution prepared by dissolving 0.270 mol of weak acid HA and 0.260 mol of its conjugate base in water sufficient to yield 1.00 L of solution.The 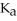 of HA is
of HA is 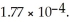
A) 2.099
B) 3.736
C) 10.264
D) 3.952
E) 2.307
Correct Answer:
Verified
Related Questions
Q66: A buffer solution with a pH of
Q67: 0.78 M Na Q68: What is the pH of a solution Q69: Consider a solution containing 0.100 M fluoride Q70: A buffer solution contains 0.100 M fluoride![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents