Cl atoms formed via photolysis of C-Cl bonds of chlorofluorocarbons in the stratosphere are particularly effective in destroying ozone at these altitudes because ________.
A) Cl atoms absorb UV, which generate O atoms to react with 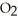 to produce ozone
to produce ozone
B) Cl atoms catalytically convert O3 to O2
C) Cl atoms stoichiometrically convert O3 to O2
D) Cl atoms react with H atoms, which catalyze conversion of O2 to O3
E) Cl atoms react with N atoms, which catalyze conversion of O2 to O3
Correct Answer:
Verified
Q5: Which of the following is released by
Q6: Of the reactions involved in the photodecomposition
Q7: Of the reactions involved in the photodecomposition
Q8: Which of the following is arranged correctly
Q9: Photoionization processes (e.g.,N2 + hν → N2+
Q11: Of the compounds below,the one that requires
Q12: Why does ozone not form in high
Q13: The source(s)of sulfur dioxide in the atmosphere
Q14: Select the substance that is thought to
Q15: Of the following,only _ does not result
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents