How does lime reduce sulfur dioxide emissions from the burning of coal?
A) It reacts with the sulfur dioxide to form calcium sulfite solid that can be precipitated.
B) It reduces the sulfur dioxide to elemental sulfur that is harmless to the environment.
C) It oxidizes the sulfur dioxide to tetrathionate that is highly water soluble so it can be scrubbed from the emission gases.
D) It catalyzes the conversion of sulfur dioxide to sulfur trioxide which is much less volatile and can be removed by condensation.
E) It converts S 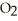 to solid, elemental sulfur.
to solid, elemental sulfur.
Correct Answer:
Verified
Q30: Incomplete combustion of carbon-containing materials occurs when
Q31: THMs are _.
A)non-toxic
B)natural
C)used in green chemistry
D)suspected carcinogens
E)atmospheric
Q32: Which of the following is classified as
Q33: Carbon dioxide contributes to atmospheric warming by
Q34: To produce acceptable quality drinking water from
Q36: Which of the following is not a
Q37: The cumulative result of carbon dioxide,methane,and ozone
Q38: Eutrophication of a lake is the process
Q39: The reaction that forms most of the
Q40: The primary detrimental effect of the presence
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents