What is the concentration (ppm) of water vapor in a sample of air that has a partial pressure of water of 1.20 torr and a total pressure of air of 695 torr?
A) 1.7
B) 0.17
C) 5.8 × 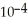
D) 1.7 × 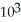
E) 0.58
Correct Answer:
Verified
Q62: What is the total pressure (torr)inside a
Q63: The "scale" caused by hard water is
Q64: What is the total pressure (torr)inside a
Q65: The concentration of ozone in a sample
Q66: What is the concentration (ppm)of helium in
Q68: Which of the following is (are)not a
Q69: The brown color of photochemical smog over
Q70: The mole fraction of nitrogen in dry
Q71: Components of Air Mole Fraction Nitrogen 0.781
Oxygen
Q72: Components of Air Mole Fraction Nitrogen 0.781
Oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents