The concentration of carbon monoxide in a sample of air is 6.0 ppm.There are ________ molecules of CO in 1.00 L of this air at 755 torr and 23 °C.
A) 2.5 × 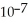
B) 1.4 × 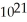
C) 1.9 × 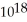
D) 1.1 × 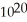
E) 1.5 × 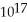
Correct Answer:
Verified
Q72: Components of Air Mole Fraction Nitrogen 0.781
Oxygen
Q73: What is the wavelength of a photon
Q74: The concentration of CO in a room
Q75: The mole fraction of nitrous oxide in
Q76: In the world's oceans,the average salinity is
Q78: The area of the Earth's atmosphere 50
Q79: What is the percentage of freshwater on
Q80: The sterilizing action of chlorine in water
Q81: What is the final stage in municipal
Q82: The stratosphere contains approximately _ of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents