Multiple Choice
The dissociation energy of N2 is 941 kJ/mol.The maximum wavelength of light that has enough energy per photon to dissociate the N2 molecule is ________ nm.
A) 3137
B) 1.27 × 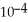
C) 1.27 × 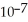
D) 1.27 × 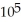
E) 127
Correct Answer:
Verified
Related Questions
Q89: Show how a molecule of C2F2Cl2 can
Q90: The absorption of a photon which results
Q91: _ and _ are major contributors to
Q92: In the equation below,what is the meaning
Q93: Which one of the following is produced
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents