The ionization energy of NO is 890 kJ/mol: NO + hv → NO+ + 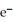 The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
A) 2967
B) 1.35 × 10-4
C) 1.35 × 10-7
D) 134.5
E) 1.35 × 105
Correct Answer:
Verified
Q79: What is the percentage of freshwater on
Q80: The sterilizing action of chlorine in water
Q81: What is the final stage in municipal
Q82: The stratosphere contains approximately _ of the
Q83: Components of Air Mole Fraction Nitrogen 0.781
Oxygen
Q85: The acidic water of a lake (V
Q86: In the presence of oxygen,the nitrogen present
Q87: Which of the following is not one
Q88: Clean rainwater is _ mainly due to
Q89: Show how a molecule of C2F2Cl2 can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents