The first law of thermodynamics can be given as ________.
A) ΔE = q + w
B) Δ 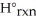 =
= 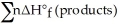 -
- 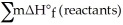
C) for any spontaneous process, the entropy of the universe increases
D) the entropy of a pure crystalline substance at absolute zero is zero
E) ΔS = 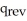 /T at constant temperature
/T at constant temperature
Correct Answer:
Verified
Q10: A reversible process is one that _.
A)can
Q11: The thermodynamic quantity that expresses the extent
Q12: Which one of the following processes produces
Q13: A reaction that is spontaneous as written
Q14: Consider a pure crystalline solid that is
Q16: Which of the following statements is true?
A)Processes
Q17: ΔS is positive for the reaction _.
A)2
Q18: The entropy of the universe is _.
A)constant
B)continually
Q19: Which of the following statements is false?
A)The
Q20: Of the following,only _ is not a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents