The equilibrium position corresponds to which letter on the graph of G vs.f (course of reaction) below? 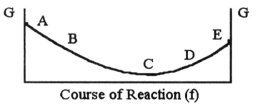
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q27: Which one of the following statements is
Q28: Which reaction produces a decrease in the
Q29: Which reaction produces an increase in the
Q30: With thermodynamics,one cannot determine _.
A)the speed of
Q31: A reaction that is not spontaneous at
Q33: A decrease in the entropy of the
Q34: The value of ΔS° for the catalytic
Q35: The combustion of acetylene in the presence
Q36: For the reaction 2 C4H10 (g)+ 13
Q37: The value of ΔS° for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents