A reaction that is not spontaneous at low temperature can become spontaneous at high temperature if 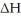 is ________ and
is ________ and 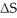 is ________.
is ________.
A) +, +
B) -, -
C) +, -
D) -, +
E) +, 0
Correct Answer:
Verified
Q26: ΔS is positive for the reaction _.
A)Pb(NO3)2
Q27: Which one of the following statements is
Q28: Which reaction produces a decrease in the
Q29: Which reaction produces an increase in the
Q30: With thermodynamics,one cannot determine _.
A)the speed of
Q32: The equilibrium position corresponds to which letter
Q33: A decrease in the entropy of the
Q34: The value of ΔS° for the catalytic
Q35: The combustion of acetylene in the presence
Q36: For the reaction 2 C4H10 (g)+ 13
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents