Consider the reaction: FeO (s) + Fe (s) + O2 (g) → Fe2O3 (s)
Given the following table of thermodynamic data, 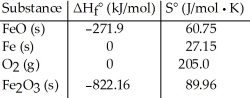 determine the temperature (in °C) at which the reaction is nonspontaneous.
determine the temperature (in °C) at which the reaction is nonspontaneous.
A) below 618.1
B) above 2438
C) above 756.3
D) below 2438
E) This reaction is spontaneous at all temperatures.
Correct Answer:
Verified
Q17: ΔS is positive for the reaction _.
A)2
Q18: The entropy of the universe is _.
A)constant
B)continually
Q19: Which of the following statements is false?
A)The
Q20: Of the following,only _ is not a
Q21: Consider the reaction: NH3 (g)+ HCl (g)→
Q23: Consider the reaction: Ag+ (aq)+ Cl- (aq)→
Q24: Given the following table of thermodynamic data,
Q25: For an isothermal process,the entropy change of
Q26: ΔS is positive for the reaction _.
A)Pb(NO3)2
Q27: Which one of the following statements is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents