Multiple Choice
Which of the following has the largest entropy at 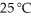 and atm?
and atm?
A) C3H4
B) C3H6
C) C3H8
D) 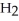
E) C2H6
Correct Answer:
Verified
Related Questions
Q79: The value of ΔG° at 25 °C
Q80: The value of ΔH° for the formation
Q81: What is the change in entropy in
Q82: Which one of the following processes produces
Q83: The normal boiling point of water is
Q85: The standard Gibbs free energy of formation
Q86: The value of ΔG° at 261.0 °C
Q87: The normal boiling point of methanol is
Q88: Which of the following has the largest
Q89: Which of the following has the largest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents