The standard cell potential (E°cell) of the reaction below is -0.34 V.The value of 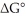 for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
A) -0.34
B) +66
C) -130
D) +130
E) none of the above
Correct Answer:
Verified
Q41: The standard cell potential (E°cell)of the reaction
Q42: The standard cell potential (E°cell)of the reaction
Q43: The less _ the value of E°red,the
Q44: In a voltaic cell,electrons flow from the
Q45: The half-reaction occurring at the anode in
Q47: The balanced half-reaction in which sulfate ion
Q48: The balanced half-reaction in which ethanol,CH3CH2OH,is oxidized
Q49: The standard cell potential (E°cell)for the voltaic
Q50: The balanced half-reaction in which dichromate ion
Q51: The reduction half reaction occurring in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents