In the electrochemical cell using the redox reaction below,the cathode half-reaction is ________. 2 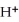 (s) + Sn (s) →
(s) + Sn (s) → 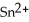 (aq) + H2 (g)
(aq) + H2 (g)
A) 2H+ + 2e- → H2
B) Sn → Sn2+ + 2e-
C) 2H+ → H2 + 2e-
D) Sn + 2e- → Sn2+
E) Sn + 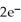 → H2
→ H2
Correct Answer:
Verified
Q60: The half-reaction occurring at the cathode in
Q61: The lead-containing reactant(s)consumed during recharging of a
Q62: In the electrochemical cell using the redox
Q63: The standard cell potential ( 
Q64: What is the correct coefficient for the
Q66: In the galvanic cell using the redox
Q67: A voltaic cell is constructed with two
Q68: Galvanized iron is iron coated with _.
A)magnesium
B)zinc
C)chromium
D)phosphate
E)iron
Q69: What is the oxidation number of nitrogen
Q70: The standard cell potential ( 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents