A voltaic cell is constructed with two silver-silver chloride electrodes,where the half-reaction is AgCl (s) + 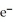 → Ag (s) +
→ Ag (s) + 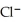 (aq) E° = +0.222 V
(aq) E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0100 M and 1.55 M,respectively.At 25 °C,the cell emf is ________ V.
A) 0.216
B) 0.130
C) 0.00143
D) 34.4
E) 0.228
Correct Answer:
Verified
Q62: In the electrochemical cell using the redox
Q63: The standard cell potential ( 
Q64: What is the correct coefficient for the
Q65: In the electrochemical cell using the redox
Q66: In the galvanic cell using the redox
Q68: Galvanized iron is iron coated with _.
A)magnesium
B)zinc
C)chromium
D)phosphate
E)iron
Q69: What is the oxidation number of nitrogen
Q70: The standard cell potential ( 
Q71: Which element is reduced in the reaction
Q72: The standard cell potential (E°)of a voltaic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents