How many seconds are required to produce 5.00 g of aluminum metal from the electrolysis of molten 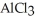 with an electrical current of 15.0 A?
with an electrical current of 15.0 A?
A) 27.0
B) 9.00
C) 1.19E × 103
D) 2.90 × 105
E) 3.57 × 103
Correct Answer:
Verified
Q93: The standard emf for the cell using
Q94: How many minutes will it take to
Q95: How many minutes will it take to
Q96: The potential (E)to move K+ from the
Q97: At constant _ and _ the Gibbs
Q99: A voltaic cell is constructed with two
Q100: How many kilowatt-hours of electricity are used
Q101: Define a coulomb.
Q102: The standard reduction potential of X is
Q103: In a half reaction,the amount of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents