How many seconds are required to produce 1.0 g of silver metal by the electrolysis of a AgN 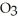 solution using a current of 60 amps?
solution using a current of 60 amps?
A) 5.4 × 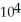
B) 3.2 × 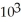
C) 15
D) 3.7 × 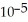
E) 30
Correct Answer:
Verified
Q79: What is the oxidation number of phosphorous
Q80: Which element is oxidized in the reaction
Q81: The standard emf for the cell using
Q82: How much time (min)will it require to
Q83: Define the Nernst equation.
Q85: How many grams of copper will be
Q86: How many grams of Cu are obtained
Q87: The electrolysis of molten Q88: How many grams of Ca metal are Q89: Based on standard reduction potentials,the most difficult![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents