Multiple Choice
How many grams of copper will be plated out by a current of 2.3 A applied for 35 minutes to a 0.50 M solution of copper (II) sulfate?
A) 1.6
B) 3.2
C) 1.8 × 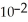
D) 3.6 × 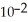
E) 0.019
Correct Answer:
Verified
Related Questions
Q80: Which element is oxidized in the reaction
Q81: The standard emf for the cell using
Q82: How much time (min)will it require to
Q83: Define the Nernst equation.
Q84: How many seconds are required to produce
Q86: How many grams of Cu are obtained
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents