What is the mass defect (in amu) of a 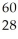 Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
A) 0.5449
B) 0.5505
C) 1.2374
D) 1.3066
E) 28.7930
Correct Answer:
Verified
Q65: In balancing the nuclear reaction 
Q66: The missing product from this reaction is
Q67: The missing product from this reaction is
Q68: Which one of the following natural radionuclides
Q69: By what process does thorium-230 decay to
Q71: What percentage of electricity generated in the
Q72: The missing reactant from this reaction is
Q73: The mass of a proton is 1.00728
Q74: Which one of the following forms of
Q75: What type of reaction is known as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents