The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.What is the mass defect (in amu) of a 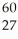 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
A) 27.7830
B) 0.5489
C) 0.5405
D) 0.0662
E) 0.4827
Correct Answer:
Verified
Q113: What drives the turbine in a nuclear
Q114: Who is credited with first achieving fission
Q115: A _ is a highly reactive substance
Q116: What is the missing product from this
Q117: What is the missing product from this
Q119: What happens to the mass number and
Q120: On average,_ neutrons are produced by every
Q121: Carbon-11 decays by positron emission.The decay occurs
Q122: What is the age in years of
Q123: The mass of a proton is 1.673
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents