Carbon-11 decays by positron emission.The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11.What mass (g) is converted to energy when 5.00 g of 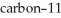 undergoes this radioactive decay?
undergoes this radioactive decay?
A) 1.45 × 10-6
B) 4.35 × 105
C) 6.90 × 102
D) 1.45 × 10-3
E) 1.59 × 10-2
Correct Answer:
Verified
Q116: What is the missing product from this
Q117: What is the missing product from this
Q118: The mass of a proton is 1.00728
Q119: What happens to the mass number and
Q120: On average,_ neutrons are produced by every
Q122: What is the age in years of
Q123: The mass of a proton is 1.673
Q124: The half-life of 218Po is 3.1 minutes.How
Q125: Cesium-131 has a half-life of 9.7 days.What
Q126: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents