The mass of a proton is 1.00728 amu and that of a neutron is 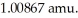 What is the mass defect (in amu) of a 57Ni nucleus? (The mass of a nickel-57 nucleus is
What is the mass defect (in amu) of a 57Ni nucleus? (The mass of a nickel-57 nucleus is 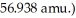
A) 0.5155 amu
B) 28.76 amu
C) -0.4932 amu
D) 0.5141 amu
E) 1.031 amu
Correct Answer:
Verified
Q145: What happens to the atomic mass number
Q146: The first nuclear transmutation resulted in the
Q147: What happens in the nucleus of an
Q148: What is the predominant isotope of uranium?
Q149: The initial element used to make cobalt-60
Q151: Control rods in a nuclear reactor are
Q152: Carbon-11,fluorine-18,oxygen-15 and nitrogen-13 are all used in
Q153: The amount of fissionable material necessary to
Q154: _ discovered radioactivity.
Q155: Stable nuclei with low atomic numbers,up to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents