Which of the following equations correctly represents the combustion of hydrazine?
A) N2H4 (l) + O2 (g) → NH3 (g) + HNO2 (g)
B) N2H4 (l) + 2O2 (g) → 2NO2 (g) + 2H2 (g)
C) N2H4 (l) + O2 (g) → 2 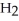 NO (g)
NO (g)
D) N2H4 (l) + O2 (g) → N2 (g) + 2 H2O (g)
E) N2H4 (l) + O2 (g) → N2 (g) + 2H2 (g) + O2 (g)
Correct Answer:
Verified
Q42: What are the products of the reaction
Q43: Carbon dioxide is produced _.
A)in blast furnaces
Q44: Which of the following is not an
Q45: Of the following substances,_ is both a
Q46: The oxidation numbers of nitrogen in the
Q48: Which of the following statements best describes
Q49: Which of the following would produce the
Q50: The reaction between nitrogen dioxide and water
Q51: Of the following,which is most likely to
Q52: How many lone pair of electrons are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents