Sodium borohydride,NaB 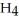 ,is a strong reducing agent because ________.
,is a strong reducing agent because ________.
A) Na+ is easily reduced to Na (s)
B) boron easily changes its oxidation number from +3 to -3
C) boron is readily oxidized from -3 oxidation state to +3
D) hydrogen can be easily oxidized from -1 oxidation state to +1
E) hydrogen is easily reduced from +1 oxidation state to 0
Correct Answer:
Verified
Q59: The oxidation number of Pb in PbO2
Q60: What is the function of the carbon
Q61: Hydrogen can form hydride ions.Elements in group
Q62: In metallic hydrides,the oxidation number of hydrogen
Q63: B2O3 is the anhydride of _.
A)borous acid
B)diborane
C)tetraboric
Q65: Boric acid condenses to form tetraboric acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents