What is the coefficient of NO2 when the following disproportionation reaction is balanced?
NO2 (g) + H2O (l) → 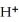 (aq) + NO3- (aq) + NO (g)
(aq) + NO3- (aq) + NO (g)
A) 1
B) 2
C) 3
D) 5
E) 4
Correct Answer:
Verified
Q89: Only the most active metals react with
Q90: The primary commercial use of oxygen is
Q91: The electron-pair geometry and molecular geometry of
Q92: The number of electrons in the valence
Q93: Br2 can be prepared by combining NaBr
Q95: What is the oxidation state of xenon
Q96: The nitride ion is a strong Br∅nsted-Lowry
Q97: Most mined phosphate rock is _.
A)used as
Q98: What is the oxidation state of xenon
Q99: The two allotropic forms of phosphorus are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents