An iron pin (specific heat = 0.473 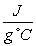 ) at 110°C is dropped into 50.0 mL of H2O at 15.0°C.After a couple of minutes,the temperature of the water reaches 17.4°C.What was the mass of the iron pin?
) at 110°C is dropped into 50.0 mL of H2O at 15.0°C.After a couple of minutes,the temperature of the water reaches 17.4°C.What was the mass of the iron pin?
A) 44 g
B) 15 g
C) 1.3 g
D) 12 g
Correct Answer:
Verified
Q79: Which term represents a form of energy?
A)Degree
B)Joule
C)Temperature
D)Heat
Q80: Select the alternative that represents a physical
Q81: In a physical change new substances are
Q82: What mass of water at 10.0°C is
Q83: Assume equal masses of the following substances,all
Q85: What will be the final temperature of
Q86: Assume equal masses of the following substances,all
Q87: Melting and boiling are examples of physical
Q88: The correct SI unit for energy is
Q89: Dry ice undergoing sublimation is an example
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents