Multiple Choice
One atom of sulfur has a mass of
A) 5.33 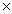 10-23 g
10-23 g
B) 32.06g
C) 1.88 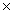 1022 g
1022 g
D) 1.93 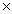 1025 g
1025 g
Correct Answer:
Verified
Related Questions
Q68: What is the molecular formula of a
Q69: What is the molecular formula of a
Q70: In which pair would both compounds have
Q71: What is the empirical formula of a
Q72: What is the molecular formula of a
Q74: In which pair would both compounds have
Q75: A 30.5 g sample of a compound
Q76: What is the molecular formula of a
Q77: What is the empirical formula of a
Q78: A compound consists of 4.50 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents