How many moles of iron(III) oxide are required to completely react with 1.40 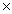 1022 atoms of carbon? Fe2O3 + 3C
1022 atoms of carbon? Fe2O3 + 3C  2Fe + 3CO
2Fe + 3CO
A) 0.0232 moles
B) 0.00775 moles
C) 0.0697 moles
D) 4.67 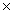 1021 moles
1021 moles
Correct Answer:
Verified
Q9: Calculate the number of moles of excess
Q10: Calculate the number of moles of excess
Q11: How many units of sodium iodide will
Q12: How many moles of NH3 will
Q13: How many moles of HF will
Q15: How many molecules of O2 will be
Q16: How many moles of molecular oxygen will
Q17: Calculate the number of moles of excess
Q18: Based on the following chemical equation 4HCN
Q19: Based on the following chemical equation 2C6H6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents