Related Questions
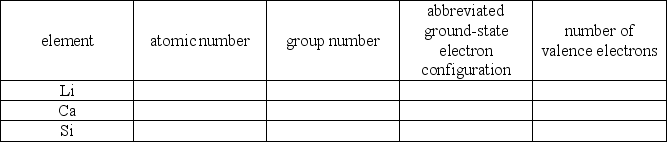
Q82: Fill the following table: Q83: The element represented by 1s2 2s2 2p1 Q84: The 4s energy sublevel fills before the Q85: An orbital represents a region around the Q86: The second principal energy level contains s Q88: How many unpaired electrons are in the Q89: Write orbital diagrams for the following elements Q90: Write orbital diagrams for the following elements Q91: In the designation 4p3;what is the significance Q92: The second principal energy level contains four
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents