The following figure represents one mole of an ideal gas in a container fit with a movable piston. 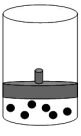 Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
A) 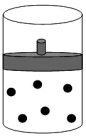
B) 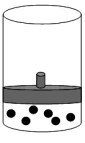
C) 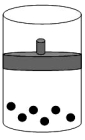
D) 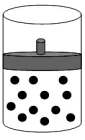
Correct Answer:
Verified
Q3: Which of the following gases would have
Q4: Which gas will diffuse most rapidly?
A)CO2
B)N2
C)He
D)Cl2
Q5: A pressure of 1030 torr is equal
Q6: A 120.mL sample of a gas is
Q7: Under which set of conditions will a
Q9: As the number of molecules in a
Q10: A 4.00 L sample of a gas
Q11: As the temperature of a gas sample
Q12: A hot air balloon is filled to
Q13: Which gas would have the lowest velocity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents