How many grams of oxygen are consumed when 18.0 L of CH4 gas react completely in the following equation? CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
A) 51.4 grams
B) 79.7 grams
C) 576 grams
D) 1.15 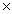 103 grams
103 grams
Correct Answer:
Verified
Q55: What volume of oxygen is consumed
Q56: At which pressure would a 2.60 mole
Q57: What is the density of carbon dioxide
Q58: Which of the following is not an
Q59: What volume of ammonia is produced
Q61: A 64.0 g sample of oxygen contains
Q62: Look at the apparatus shown below.Inside each
Q63: Which gas is most dense at STP?
A)Helium
B)Nitrogen
C)Oxygen
D)Hydrogen
Q64: What is the volume occupied by 25.0
Q65: Calculate the density of argon gas at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents