A solution of a strong base has a pH of 11.The concentration of hydronium ions in this solution is
A) 11 M
B) 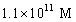
C) 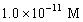
D) 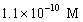
Correct Answer:
Verified
Q52: Which is a conjugate acid base pair
Q53: Which are the two Bronsted-Lowry acids in
Q54: What is the conjugate base of NH3?
A)NH2
Q55: Which are the two Bronsted-Lowry acids in
Q56: Which is a conjugate acid base pair
Q58: What is the conjugate base of HS
Q59: Which are the two Bronsted-Lowry bases in
Q60: Which type of mixture displays Brownian motion
Q61: What is the pH of a 0.01
Q62: What is the freezing point of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents