True/False
The net ionic equation for the neutralization of nitric acid by potassium hydroxide is 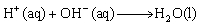 .
.
Correct Answer:
Verified
Related Questions
Q76: What mass of ethanol,C2H5OH,should be added to
Q77: What mass of sodium chloride should be
Q78: A solution is prepared by adding 35.5
Q79: What is the pH of a 0.020
Q80: What is the freezing point of a
Q82: A 0.1 M HNO3 solution and a
Q83: At 25 °C the concentration of H
Q84: A hydronium ion is a hydrated proton.
Q85: The pH of an aqueous solution of
Q86: Strong acids are strong electrolytes.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents