The solubility of silver bromide is 7.1 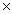 10-7 M.The Ksp of silver bromide is
10-7 M.The Ksp of silver bromide is
A) 7.1 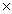 10-5
10-5
B) 5.0 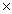 10 -5
10 -5
C) 5.0  10-13
10-13
D) 5.0 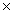 10-15
10-15
Correct Answer:
Verified
Q69: What will be the [H+1] in a
Q70: The mathematical expression for the equilibrium constant
Q71: The sodium salts of all anions listed
Q72: What is the pH of a 0.00500
Q73: The concentration of hydroxide ions, 
Q75: The solubility of CaF2 is 2.14
Q76: A 0.20 M solution of the weak
Q77: What is the pH of 0.50 M
Q78: In the following equilibrium;as the pressure is
Q79: If HCl (aq)is added to a saturated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents