Calculate the nuclear binding energy for 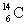 using the following information: proton mass = 1.0073 g/mol
using the following information: proton mass = 1.0073 g/mol
Neutron mass = 1.0087 g/mol
Electron mass = 0.00055 g/mol 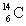 = 14.0032 g/mol
= 14.0032 g/mol
1) 0 g = 9.0  1013 J
1013 J
A) 2.9  1011 J/mol
1011 J/mol
B) 9.9 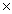 1012 J/mol
1012 J/mol
C) 7.6 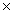 1012 J/mol
1012 J/mol
D) 8.5 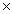 1012 J/mol
1012 J/mol
Correct Answer:
Verified
Q11: All nuclides of which element must be
Q12: The mass of an alpha particle is
A)1
Q13: The charge of an alpha particle is
A)+1
B)+2
C)-1
D)0
Q14: Which particles are deflected towards the positive
Q15: Nuclei with atomic number up to about
Q17: A nucleon is
A)the sum of all the
Q18: The instrument used to measure ionizing radiation
Q19: An alpha particle consists of
A)one proton and
Q20: Which particles are deflected towards the negative
Q21: I-131 decays by beta decay to produce
A)Te-131
B)Sb-127
C)Xe-131
D)Cs-135
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents